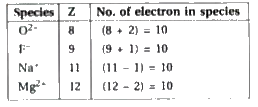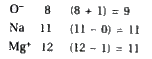A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
KUMAR PRAKASHAN|Exercise Section -C(MCQ asked in Board Exam )|53 VideosSTRUCTURE OF ATOM
KUMAR PRAKASHAN|Exercise SECTION -D (MULTIPLE CHOICE QUESTIONS)|55 VideosSTRUCTURE OF ATOM
KUMAR PRAKASHAN|Exercise Section -C(MCQs asked in Competitive Exam)|69 VideosSTATES OF MATTER
KUMAR PRAKASHAN|Exercise QUESTION PAPER FROM MODULE (SECTION - A)|4 VideosTHE P-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION-D NCERT EXEMPLAR SOLUTION (MCQs) LONG ANSWER|25 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-STRUCTURE OF ATOM-Section -C (MCQs asked in Jee NEET AIEEE )
- The electron identified by quantum number n and l
Text Solution
|
- The value of plank constant is 6.63 xx 10^(10^(34) kg the speeds ...
Text Solution
|
- What is the maximum number of electrons that can be associated with th...
Text Solution
|
- Energy of an electron is given by E=-2.718*10-18 J Wavelength of light...
Text Solution
|
- the correct set of the four quantum number for the valence electro...
Text Solution
|
- What is the maximum number of orbitals that can be identified with the...
Text Solution
|
- Calculate the energy in corresponding to light of wavelength 45 nm. (P...
Text Solution
|
- What is the maximinn number of orbitals that can be identified with th...
Text Solution
|
- Based on equation E=-2.178 xx10^(-15)J (z^(2))/(n^(2)) certain conclu...
Text Solution
|
- The angular m om entum of electron in ‘d ’ orbital is equal to
Text Solution
|
- Which of the following is the energy of a possible excited state of hy...
Text Solution
|
- A stream of electrons from a heated filament was passed between two ch...
Text Solution
|
- Two electrons occupying the same orbital are distinguished by
Text Solution
|
- How many electrons can fit in the orbital for which n = 3 and Z = 1 ?
Text Solution
|
- Which of the following pairs of d-orbitals will have electron density ...
Text Solution
|
- The energies E(1) and E(2) respectively. The relation between their wa...
Text Solution
|
- The frequency transition n = 4 to n = 2 of He+ is equal to the transit...
Text Solution
|
- (e )/(m) ratio was determined by
Text Solution
|
- The group having isoelectronic species i s ....
Text Solution
|
- The total num ber of orbitals present for principle quantum number n =...
Text Solution
|