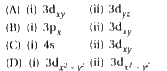A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOM
KUMAR PRAKASHAN|Exercise QUESTINS PAPER FROM MODULE (SECTION -A)|6 VideosSTRUCTURE OF ATOM
KUMAR PRAKASHAN|Exercise QUESTINS PAPER FROM MODULE (SECTION -B)|3 VideosSTRUCTURE OF ATOM
KUMAR PRAKASHAN|Exercise Section -C(MCQ asked in Board Exam )|53 VideosSTATES OF MATTER
KUMAR PRAKASHAN|Exercise QUESTION PAPER FROM MODULE (SECTION - A)|4 VideosTHE P-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION-D NCERT EXEMPLAR SOLUTION (MCQs) LONG ANSWER|25 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-STRUCTURE OF ATOM-SECTION -D (MULTIPLE CHOICE QUESTIONS)
- If travelling at same speeds, which of the following matter waves have...
Text Solution
|
- Identify the pairs which are not of isotopes
Text Solution
|
- Out of the following pairs of electrons, identify the pairs of electro...
Text Solution
|
- Which of the following sets of quantum numbers are correct ?
Text Solution
|
- In which of the following pairs, the ions are iso electro n ic ?
Text Solution
|
- Which of the following statements concerning the quantum numbers are c...
Text Solution
|
- Arrange s, p an d subshells of a shell in the increasing order of effe...
Text Solution
|
- Show the distribution of electrons in oxygen atom (atom ic num ber 8) ...
Text Solution
|
- Nickel atom can lose two electrons to form Ni^(2+) ion. The atomic num...
Text Solution
|
- Which of the following orbitals are degenerate ? 3d(xy), 4d(xy), 3d(z^...
Text Solution
|
- Calculate the total number of angular nodes and radial nodes present i...
Text Solution
|
- The arrangemen t of orbitals on the basis of energy is based upon th e...
Text Solution
|
- Which of the following will not show deflection from the path on passi...
Text Solution
|
- An atom having atom ic m ass num ber 13 has 7 neutrons. W hat is the a...
Text Solution
|
- Wavelengths of different rad iatio n s are given below : lambda(A) - 3...
Text Solution
|
- Show the distribution of electrons in oxygen atom (atom ic num ber 8) ...
Text Solution
|
- The Balm er series in the hydrogen sp ectru m corresponds to the trans...
Text Solution
|
- According to de Broglie, matter should exhibit dual behaviour, that is...
Text Solution
|
- What is the experimental evidence in support of the idea that electron...
Text Solution
|
- Out of electron and proton which one will have, a higher velocity to p...
Text Solution
|