Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOM
KUMAR PRAKASHAN|Exercise QUESTINS PAPER FROM MODULE (SECTION -A)|6 VideosSTRUCTURE OF ATOM
KUMAR PRAKASHAN|Exercise QUESTINS PAPER FROM MODULE (SECTION -B)|3 VideosSTRUCTURE OF ATOM
KUMAR PRAKASHAN|Exercise Section -C(MCQ asked in Board Exam )|53 VideosSTATES OF MATTER
KUMAR PRAKASHAN|Exercise QUESTION PAPER FROM MODULE (SECTION - A)|4 VideosTHE P-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION-D NCERT EXEMPLAR SOLUTION (MCQs) LONG ANSWER|25 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-STRUCTURE OF ATOM-SECTION -D (MULTIPLE CHOICE QUESTIONS)
- A hypothetical electromagnetic wave is shown in Figure. Find out the w...
Text Solution
|
- Chlorophyll present in green leaves of plants absorbs light at 4.620 x...
Text Solution
|
- What is the difference between the terms orbit and orbital ?
Text Solution
|
- Table-tennis ball has a mass 10 g and a speed of 90 m/s. If speed can ...
Text Solution
|
- The effect of uncertainty principle is significant only for motion of ...
Text Solution
|
- Hydrogen atom has only one electron, so mutual repulsion between elect...
Text Solution
|
- Match the following species with their corresponding ground state ele...
Text Solution
|
- Match the quantum numbers with the information provided by these
Text Solution
|
- Match the following rules with their statements
Text Solution
|
- Match the following
Text Solution
|
- Match the following
Text Solution
|
- Match species given in Column-I with the electronic configuration give...
Text Solution
|
- Assertion (A) : All isotopes of a given element show the same type of ...
Text Solution
|
- Assertion (A) : Black body is an ideal body that emits and absorbs rad...
Text Solution
|
- Assertion (A) : It is impossible to determine the exact position and e...
Text Solution
|
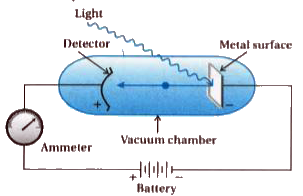
- What is photoelectric effect ? State the result of photoelectric effec...
Text Solution
|
- Threshold frequency, v(0) is the minimum frequency which a photon must...
Text Solution
|
- When an electric discharge is passed through hydrogen gas, the hydroge...
Text Solution
|
- Calculate radiation from n = 3 t o n = 2 i n a hydrogen atom.
Text Solution
|
- Why was a change in the Bohr Model of atom required ? Due to which imp...
Text Solution
|