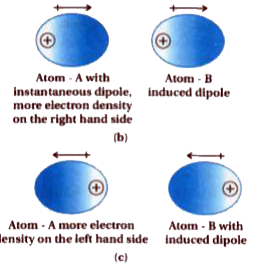
Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER
KUMAR PRAKASHAN|Exercise SECTION - A QUESTIONS (Sub Question)|3 VideosSTATES OF MATTER
KUMAR PRAKASHAN|Exercise SECTION - A QUESTIONS (TRY YOUR SELF)|47 VideosSOME BASIC CONCEPTS OF CHEMISTRY
KUMAR PRAKASHAN|Exercise Section - D (Solutions of NCERT Exemplar Problems) (Long Answer Type Questions)|4 VideosSTRUCTURE OF ATOM
KUMAR PRAKASHAN|Exercise QUESTINS PAPER FROM MODULE (SECTION -D)|2 Videos
Similar Questions
Explore conceptually related problems