Charles. law and Formula : Charles. and Gay Lussac performed several experiments on gases independently to imporove upon hot air balloon technology.
Simple Gay Lussac.s law : Their investigations showed that for a fixed mass of a gas at constnat pressure, volume of a gas increases on increasing temperature and decreases on cooling.
Charles and Gay Lussac they found that - ..for each degree rise in temperature, volume of a gas increases by `(1)/(273.15)` of the original volume `(V_(0))` of the gas at `0^(@)C`. ..
Thus, temperature volume `0^(@)C=V_(0)` and
temperature volume `t^(@)C=V_(t)` then,
`V_(t)=V_(0)+((t)/(273.15))V_(0)`
`therefore V_(t)=V_(0)(1+(t)/(273.15)) " "` ......(Eq. -i)
`therefore V_(t)=V_(0)((273.15+t)/(273.15)) " "` ....(Eq. -ii)
But `(273.15+t)""^(@)C=T_(1)k`
So, `273.15^(@)C=T_(0)=273.15k`
Thus `V_(t)=V_(0)((T_(1))/(T_(0))) " "` ....(Eq. -iii)
`therefore (V_(t))/(V_(0))=(T_(1))/(T_(0)) " "` ....(Eq. -iv)
OR `(V_(2))/(V_(1))=(T_(2))/(T_(1)) " "` ....(Eq. - v)
So, `(V_(2))/(T_(2))=(V_(1))/(T_(1))" "` ...(Eq. - vi)
Thus `(V)/(T=` constant `k_(2) " "` .....(Eq. -vii)
`therefore V=k_(2)T " "` .....(Eq. - viii)
This (Equation - viii) is the mathermatical expression for Charles. law.
Charles. law : He states that pressure remaining constant, the volume of a fixed mass of a gas is directly proportional to its absolute temperature.
Characteristics and Graph of Charle.s Law :
For all gases, at any given pressure, graph of volume vs remperature (in celsius) is as under.
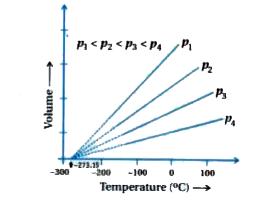
the graph is a straight line.
the graph extending to zero volume.
Slopes of lines obtained at different pressure are differnt `(p_(1), p_(2), p_(3),.....)`.
Each line intercepts the temperature axis at `- 273.15^(@)C`.
But at zero volume all the lines meet the temperature axis at `- 273.15^(@)C`.
All the lines are isobar because pressure is constant.
