Pressure in well inflated tyres of automobiles is almost constant, but on a hot summer day tyre may burst if pressure is not adjusted properly. During winters on a cold morning one may find the pressure in the tyres of a vechicle decreased considerably.
Law : At constant volume, pressure of a fixed amount of a gas varies directly with the temperature.
The rule as mathematically :
`p prop T` (at constant V) ....(Eq. - i)
and `p=K_(3)T` (at constant V) ....(Eq. -ii)
So, `(p)/(T)=K_(3)=` constant .....(Eq.-iii)
Law : ..At constant volume ratio of pressure and absolute temperature of gas is constant...
Formula of changes of temperature and pressure at constant volume : Suppose, at constant volume initial pressure is `p_(1)` and initial temperature `T_(1)` and final pressure is `p_(2)` and final temperature is `T_(2)`.
According to Gay Lussac.s Law, `(p_(1))/(T_(1))=k_(3)=(p_(2))/(T_(2))`
Thus, `(p_(1))/(T_(1))=(p_(2))/(T_(2)) " "` ....(Eq. -iv)
and `(p_(1))/(p_(2))=(T_(1))/(T_(2)) " "` ....(Eq. -v)
and `p_(1)T_(2)=p_(2)T_(2) " "` .....(Eq. - vi)
Isocore Graph : Pressure vs temperature (Kelvin) graph at constant molar volume is shown in Fig.
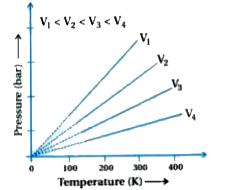
The graph is a strait line with positive slope.
Each line of different volume `(V_(1),V_(2),V_(3),V_(4))` are isochore.
All the isohores expanded to low temperature are gathered at origin point.
`therefore` At zero K temperature, pressure is zero therefore at zero K temperature there is no gaseous state.
We can get Gay Lussac.s law by using Boyle.s and Charle.s law.
