First complete data on pressure - volume - temperature relations of a substance in both gaseous and liquid state was obtained by Thomas Andrews on carbon dioxide.
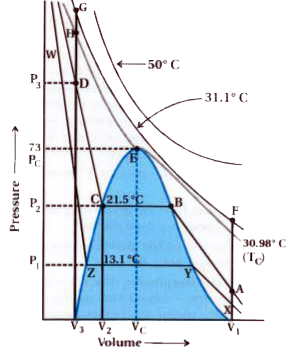
From graph derivations of Andrews : (ii) As the temperature is lowered, shape of the curve changes and data shows considerable deviation from ideal behaviour.
(iii) Explanation of `T_(C )`, (E point) `P_(C )` and `V_(C )` : At `30.98^(@)C` carbon dioxide remains gas upto 73 atmospheric pressure. (Point E in Figure). At 73 atmospheric pressure, liquid carbon dioxide appears for the first time.
Critical temperature `(T_(C )) : 30.98^(@)C` temperature is known as critical temperasture of `CO_(3)` gas. While firct pressure of `CO_(2)` gas becomes liquid at 73 atoms.
Higher than `30.98^(@)C CO_(2)` gas is in gaseous from `(T_(C ))`.
At critical temperature volume of 1 mole gas is known as critical volume `(V_(C ))` and pressure is known as critical pressure `(P_(C ))`.
Critical pressure : The critical temperature `(T_(C ))`, critical pressure `(P_(C ))` and critical volume `(V_(C ))` are called critical constants.
`30.98^(@)C` further increase in pressure simply compresses the liquid carbon dioxide and the curve represents the compressibility of the liquid. The steep line represents the isotherm of liquid. Even a slight compression results in steep rise in pressure indicating very low compressibility of the liquid.
Below `30.98^(@)C (T_(C ))` or (`21.5^(@)C` point B) and BC :
Below `30.98^(@)C` the behasviour of the gas on compression is quite different.
At `21.5^(@)C`, carbon dioxide remains as a gas only upto point B.
Further compression does not chage the pressure. - Liquid and gaseous carbon dioxide coexist and further application of pressure results in the condensation of more gas until the point C is reached.
At point C, all the gas has been condensed and further application of pressure merely compresses the liquid as shown by steep line.
A slight compression from volume `V_(2)` to `V_(3)` results in steep rise in pressure from `P_(2)` to `P_(3)`.
Below `30.98^(@)C` (criticasl temperature) each curve shows the similar trend and only length of the horizontal line increases at lower temperatures. (At E point, after BC line length is increases.)
At critical point horizontal portion of the isotherm merges into one point. In figure : (i) Point A : Represents gaseous state (ii) Point D : Represent liquid state. (iii) Dome shaped area represents existence of liquid and gaseous carbon dioxide in equilibrium.
