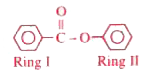A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the compound Electrophilic substitution occurs rapidly at
Text Solution
|
- In a compound electrophilic substitution has occurred the substituents...
Text Solution
|
- Electrophilic substitution in phenol generally occurs at
Text Solution
|
- Electrophilic substitution in the following compound will be fastest a...
Text Solution
|
- The compound electrophilic substitution has occurred The substituent ...
Text Solution
|
- हैलोऐरीनस में इलेक्ट्रोफिलिक प्रतिस्थापन o- व p- स्थानों पर ही क्यों ह...
Text Solution
|
- फीनॉल में इलेक्ट्रोफिलिक प्रतिस्थापन केवल आर्थों एवं पैरा स्थान पर होत...
Text Solution
|
- The most reactive compound in electrophilic substitution reaction is
Text Solution
|
- The most reactive compound towards electrophilic substitution reaction...
Text Solution
|