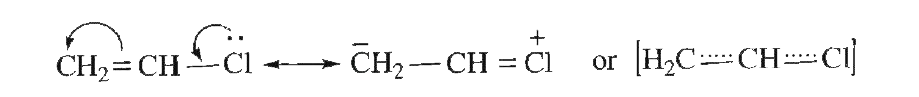Text Solution
Verified by Experts
Topper's Solved these Questions
ALKYL AND ARYL HALIDES
AAKASH SERIES|Exercise Exercise-1.1.1|6 VideosALKYL AND ARYL HALIDES
AAKASH SERIES|Exercise Exercise-1.1.2|14 VideosALKYL AND ARYL HALIDES
AAKASH SERIES|Exercise PRACTICE EXERCISE|52 VideosALDEHYDES AND KETONES
AAKASH SERIES|Exercise Exercise 3.2|41 VideosAMINES AND AZO COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 6 (Integer answer type Questions)|9 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ALKYL AND ARYL HALIDES-Problem
- Expain the formation of the two products in the following reaction : ...
Text Solution
|
- Predict the major product obtained by dehydrochlorination of
Text Solution
|
- Why the chlorine atom in vinyl chloride is nonreactive?
Text Solution
|
- Allyl iodide can be obtained from allyl chloride. Explain.
Text Solution
|
- Write the structures of major and minor products formed when 3-chloro-...
Text Solution
|
- Arrange each set of compounds in order of increasing boiling points ...
Text Solution
|
- Draw the structures of major products in each of the following reactio...
Text Solution
|
- 2-Bromo-2, 3-dimethylbutane is treated with alcoholic potash. Write th...
Text Solution
|
- In the following pairs of halogen compounds which would undergo S(N^(2...
Text Solution
|
- Chloroform is treated with aqueous silver nitrate. What happens?
Text Solution
|
- How do you distinguish between CH(3)CH=CHCl, CH(3)CH(2)CH(2)Cl and CH(...
Text Solution
|
- How iodoform is distinguished from chloroform?
Text Solution
|
- How will you distinguish between chloroform and carbon tetrachloride ?
Text Solution
|
- What is teflon ? How is it prepared ?
Text Solution
|
- What are freons? How are they prepared?
Text Solution
|
- Among the three isomeric dichlorobenzenes , which has the highest...
Text Solution
|
- Benzyl chloride undergoes nucleophilic substitution much more easily t...
Text Solution
|
- Which product will form when optically active form of C4 H9 Br is subj...
Text Solution
|
- Nucleophilic substitution in aryl halides is facilitated by electron w...
Text Solution
|
- Identify A, B, C, D, E, R and R' in the following R-Br + Mg overs...
Text Solution
|