Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTRON MIGRATION EFFECTS
AAKASH SERIES|Exercise EXERCISE 2 . 1.1|4 VideosELECTRON MIGRATION EFFECTS
AAKASH SERIES|Exercise EXERCISE 2 . 1.2|5 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise PRACTICE EXERCISE|50 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II (PRACTICE SHEET ADVANCE)) (Integer Type Questions)|3 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ELECTRON MIGRATION EFFECTS -QUESTIONS FOR DESCRIPTIVE ANSWERS
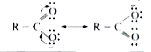
- Why the bond length of two C,O bonds in carboxylate ions are equal?
Text Solution
|
- Hyperconjugation effect is also termed as 'no bond resonance'. Why?
Text Solution
|
- Explain why alkyl groups act as electron donors when attached to a π s...
Text Solution
|
- Separate nucleophiles and electrophiles from: H(3)O^(+), RCOO^(-), BF(...
Text Solution
|
- Classify the following reactions in one of the reaction type : (i) C...
Text Solution
|
- Why trichloro acetic acid is more reactive than acetic acid ?
Text Solution
|
- Among the ions, O(2)N CH(2)CH(2)O^(-) and CH(3) CH(2)O^(-) , which is ...
Text Solution
|
- C(2)H(5)^(+) is more stable than CH,+. Substantiate ?
Text Solution
|
- What type of reaction is involved in the conversion of ammonium cyanat...
Text Solution
|
- Halogen causes –I effect, buit with lone pairs they have +M effect. Su...
Text Solution
|
- Phenol is more acidic and ethanol is less acidic than water. Why ?
Text Solution
|