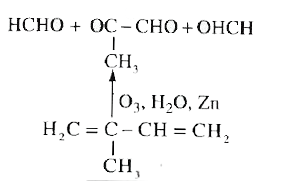
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ALIPHATIC HYDROCARBONS-QUESTIONS FOR DESCRIPTIVE ANSWERS
- Isoprene is a diene. How are the positions of double bonds located?
Text Solution
|
- C(2)H(5)Clunderset(KOH)overset(Alc)toAunderset(C Cl(4))overset(Br(2))t...
Text Solution
|
- Among aliphatic hydrocarbons, why alkanes are less reactive?
Text Solution
|
- Propene reacts with HBr to give isopropyl bromide but not n-propyl bro...
Text Solution
|
- A gaseous mixture contains ethylene and acetylene. How are they separa...
Text Solution
|
- How is unsaturation in organic compounds tested ?
Text Solution
|
- A gaseous mixture contains ethane and ethylene. How are the individual...
Text Solution
|
- Propanal and propanone are the ozonolysis products of an alkene. Wžite...
Text Solution
|
- Name the product of addition of sulphur monochloride to ethylene? What...
Text Solution
|
- Various products formed when ethylene reacts with concentrated sulphur...
Text Solution
|
- Give the equation for the reaction between 1-butene and chlorine water...
Text Solution
|
- Acetylene gives a compound 'X' when treated with water in the presence...
Text Solution
|
- Write IUPAC names of the products obtained by the ozonolysis of the fo...
Text Solution
|
- Only propanone is formed during the ozonolysis of an alkene ? Give its...
Text Solution
|
- Write the major and minor products formed when 1-butene adds on HBr in...
Text Solution
|
- Give the structural formula of the product formed when n-propyl alcoho...
Text Solution
|
- Write the IUPAC names of the products obtained by the ozonolysis of th...
Text Solution
|
- Which of the following would exhibit geometrical isomerism ? Hex-2-ene...
Text Solution
|
- An alkene 'A' contains three C-C, eight C-H bonds and one C=C bond. 'A...
Text Solution
|
- Among ethylene and acetylene, which is more reactive towards electroph...
Text Solution
|
- Differentiate between linear polymerisation and cyclic polymerisation ...
Text Solution
|