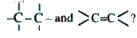Topper's Solved these Questions
AROMATIC HYDROCARBONS
AAKASH SERIES|Exercise Exercise 5.1.2|7 VideosAROMATIC HYDROCARBONS
AAKASH SERIES|Exercise Exercise 5.1.3|5 VideosAROMATIC HYDROCARBONS
AAKASH SERIES|Exercise Problems|21 VideosALKALINE EARTH METALS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE ADDITIONAL QUESTIONS|15 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|20 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-AROMATIC HYDROCARBONS-Exercise 5.1.1
- Write the characteristics of aromatic compounds.
Text Solution
|
- State Hucel's rule and explain.
Text Solution
|
- Resonance energy of benzene is
Text Solution
|
- Explain the orbital model structure of benzene.
Text Solution
|
- Explain the aromatic character of naphthalene and anthracene.
Text Solution
|
- Why the bond distance in benzene is inter-mediate between
Text Solution
|