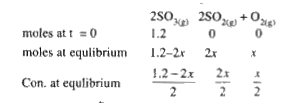A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 1.2 moles of SO(3) are allowed to dissociate in a 2 litre vessel the ...
Text Solution
|
- A reaction system in equilibrium according to reaction 2SO(2)(g)+O(2)(...
Text Solution
|
- In a closed container at 1 atm pressure, 2 mole of SO(2) (g) and 1 mol...
Text Solution
|
- A vessel of one litre capacity containing 1 mole of SO(3) is heated ti...
Text Solution
|
- The equilibrium constant (Kc) for the following equilibrium 2So(2(g)) ...
Text Solution
|
- At equilibrium degree of dissociation of SO(3) is 50% for the reaction...
Text Solution
|
- One mole of SO(3) was placed in a litre reaction vessel at a certain t...
Text Solution
|
- For the reaction : 2 NH(3)(g) rarr N(2)(g) + 3 H(2)(g)
Text Solution
|
- 10 moles of SO(2) and 4 moles of O(2) are mixed in a closed vessel of ...
Text Solution
|