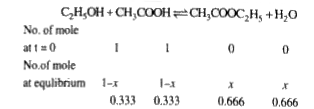A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 1.0 mole of ethyl alcohol and 1.0 mole of acctic acid are mixed. At eq...
Text Solution
|
- One mole of ethanol is treated with one mole of ethanoic acid at 25^(@...
Text Solution
|
- If 1 mole of acetic acid and 1 mole of ethyl alchol are mixed and rea...
Text Solution
|
- यदि एक मोल ऐसीटिक अम्ल और 1 मोल एथिल एलकोहॉल आपस में अभिक्रिया कर साम्...
Text Solution
|
- One mole of ethanol is treated with one mole of ethanoic acid at 25^@C...
Text Solution
|
- If 1 mole of acetic acid and 1 mole of ethyl alcohol are mixed togethe...
Text Solution
|
- 1.0 mole of ethyl alcohol and 1.0 mole of acctic acid are mixed. At eq...
Text Solution
|
- 25°C पर 1 मोल एसिटिक अम्ल के साथ ऐथिल एल्कोहल के एक मोल की क्रिया कराय...
Text Solution
|
- One mole of pure ethyl alcohol was treated with one mole of pure aceti...
Text Solution
|