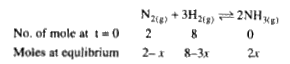A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A mixture of 2 moles of N(2) and 8 moles of H(2) are heated in a 2 lit...
Text Solution
|
- For the reaction, N(2)+3H(2)hArr2NH(3), in a vessel equal moles of N(2...
Text Solution
|
- For the reaction: N(2)+3H(2)hArr2NH(3) If the initial concentratio...
Text Solution
|
- 1 mole N(2) and 3 moles of H(2) are taken in a 4 litre vessel and heat...
Text Solution
|
- One mole of H(2) and 2 moles of I(2) are taken initially in a two lit...
Text Solution
|
- 1 "mole of" N(2) "and" 3 "moles of " H(2) are placed in 1L vessel. Fin...
Text Solution
|
- One mole of H(2) and mole of I(2) are allowed to attain equilibrium in...
Text Solution
|
- 1 mole of N(2) and 2 moles of H(2) are allowed to react in a 1 dm^(3) ...
Text Solution
|
- A mixture of 2 moles of N(2) and 8 moles of H(2) are heated in a 2 lit...
Text Solution
|