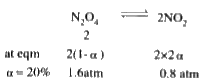A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- N2O4 at an initial pressure of 2 atm and 300K dissociates to an extent...
Text Solution
|
- For the reaction N2O4 hArr 2NO2, at 350 K, the value of Kc =0.4.The va...
Text Solution
|
- The equilibrium system, N2O4(s) hArr 2NO2(g) , has Kp=11 .For which eq...
Text Solution
|
- 138 gm N2O4 is introduced into 8.21 litre container at 300 K. Temperat...
Text Solution
|
- N2O4(g) hArr 2NO2(g) . In this reaction, NO2 is 20% of the total volum...
Text Solution
|
- Kp for the reaction N2O4 (g) hArr 2NO2(g) is 0.66 at 46^@C. Calculate ...
Text Solution
|
- AT constant temperature, the equilibrium constant (Kp) for the decompo...
Text Solution
|
- Kp for the reaction : N2O4(g) hArr 2NO2(g) is 0.157 atm at 27^@C an...
Text Solution
|
- At 800K, Kp for the reaction: N2O4 hArr 2NO2 is 700 mm. Calculate ...
Text Solution
|