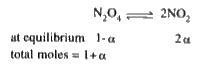A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the reaction N2O4 harr 2NO(2(g)), the degree of dissociation at eq...
Text Solution
|
- Gaseous N2O4 dissociates into gaseous NO2 according to the reaction N2...
Text Solution
|
- For the dissociation reaction N(2)O($) (g)hArr 2NO(2)(g), the degree o...
Text Solution
|
- For the reaction N(2)O(4)(g) hArr 2NO(2)(g) the degree of dissociation...
Text Solution
|
- For the reaction N(2)O(4)hArr 2NO(2(g)), the degree of dissociation of...
Text Solution
|
- For the reaction , N2O4(g)hArr2NO2(g) the degree of dissociation at eq...
Text Solution
|
- For the reaction, N(2)O(4)(g)hArr 2NO(2)(g) the degree of dissociation...
Text Solution
|
- At 800K, Kp for the reaction: N2O4 hArr 2NO2 is 700 mm. Calculate ...
Text Solution
|
- Give the equilibrium N2O4(g) hArr 2NO(2)(g) wih Kp=0.15 atm at 298 K...
Text Solution
|