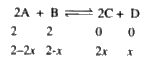A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the gaseous phase reaction 2A+B harr 2C+D, initially there are 2 m...
Text Solution
|
- For the reaction, A+B hArr 2C, 2 mol of A and 3 mol of B are allowed t...
Text Solution
|
- The equilibrium constant for the reaction 2A+2B hArr 2C+2D is 200 . Th...
Text Solution
|
- A+3B harr 2C+D For this hypothetical reaction, initial moles of A is t...
Text Solution
|
- 4 moles of A are mixed with 4 moles of B. At equilibrium for the racti...
Text Solution
|
- गैसीय अभिक्रिया A + 2B hArr 2C + D में B का प्रारंभिक सांद्रण A का 1.5...
Text Solution
|
- In the following hypothetical reaction A+3B hArr 2C+D initial moles of...
Text Solution
|
- For the reaction A+3B hArr 2C+D initial mole of A is twice that of B ....
Text Solution
|
- For reaction 2A+B hArr 2C, K=x Equilibrium constant for C hArr A+1//2 ...
Text Solution
|