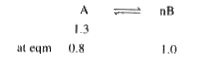A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
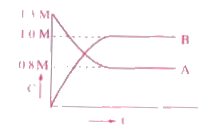
- As per the graph what is Kc of A harr nB
Text Solution
|
- The relationship between Kp and Kc is Kp=Kc(RT)^(Deltan) . What would ...
Text Solution
|
- For a reaction N2+3H2 hArr 2NH3 , the value of Kc would depend upon
Text Solution
|
- For PCI5(g) hArr PCI3(g) +CI2(g) , write the expression of Kc .
Text Solution
|
- For 2HI(g) hArr H2(g)+I2(g) write the expression of kc .
Text Solution
|
- At 500 K, Kc for the reaction: H2(g) + D2(g) hArr 2HD (g) is 3.6 W...
Text Solution
|
- দেওয়া আছে : (i) A hArr B + C , Kc = 2 , (ii) C hArr B + D , Kc = 3 ,...
Text Solution
|
- A + 2B hArr C এবং C hArr 2D বিক্রিয়াদ্বয়ের এর ক্ষেত্রে Kc এর মান যথ...
Text Solution
|
- KC/KP for the reaction, N2(g) + 3H2(g) hArr 2NH3(g) is ……………………. .
Text Solution
|