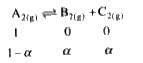A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The equilibrium constant K (in atm) for the reactio is 9 at 7 atm and ...
Text Solution
|
- The equilibrium constant for the reaction A(2)(g)+B(2)(g) hArr 2AB(g) ...
Text Solution
|
- If the equilibrium constant for the reaction A(2)+B(2) hArr 2AB is...
Text Solution
|
- The equilibrium constant of the reaction A(2)(g)+B(2)(g) hArr 2AB(g) a...
Text Solution
|
- For the dissociation A(2)B(3)hArr2AB(g) + (1)/(2)B(2)(g) If, M = M...
Text Solution
|
- A sealed container was filled with 1 mol of A(2)(g) 1 mol B(2)(g) at 8...
Text Solution
|
- Equimolar mixture of two gases A(2) and B(2) is taken in a rigid vesse...
Text Solution
|
- The equilibrium constant (K(c)) of the reaction A(2)(g) + B(2)(g) rarr...
Text Solution
|
- For the Chemical reaction A(2(g)) + B(2(g)) hArr 2 AB(g) the amount o...
Text Solution
|