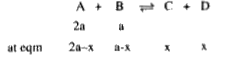A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In an equilibrium A+BhArrC+D,A and B are mixed in a vessel at temperat...
Text Solution
|
- In an equilibrium A+B hArr C+D, A and B are mixed in vessel at tempera...
Text Solution
|
- For the reaction A+B hArr C+D , the initial concentrations of A and B ...
Text Solution
|
- For the reaction, A+BhArr C+D, the initial concentration of A and B ar...
Text Solution
|
- अभिक्रिया A + B ltimpliesC + D में A की आरंभिक सांद्रता B की सांद्रता ...
Text Solution
|
- For the reaction a+bhArrc+d , initially concentrations of a and b are ...
Text Solution
|
- In an equilibrium A+BhArrC+D,A and B are mixed in a vessel at temperat...
Text Solution
|
- For A+BhArrC, the equilibrium concentrations of A and B at a temperatu...
Text Solution
|
- In the reaction, A+BhArrC+D in a one - litre container, concentration ...
Text Solution
|