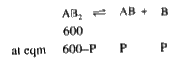A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- AB(2(g)) dissociates as AB(2(g)) harr AB(2(g))+B((g)). The initial pre...
Text Solution
|
- AB(2) dissociates as AB(2)(g) hArr AB(g)+B(g) . If the initial pressur...
Text Solution
|
- AB(3)(g) is dissociation as AB(2)(g) hArr AB(2)(g)+(1)/(2)B(2)(g), W...
Text Solution
|
- AB(3)(g) is dissociates as AB(3)(g)hArrAB(@)(g)+(1)/(2)B(@)(g) When th...
Text Solution
|
- AB(2) " dissociates as " : AB(2) (g) hArr AB (g) + B (g). If the intia...
Text Solution
|
- The dissociation equilibrium of AB(2) gas is, 2AB(2)(g) hArr 2AB(g)+...
Text Solution
|
- XY2 dissociates as XY2 (g) hArr XY(g) +Y(g) Initial pressure XY2 is 60...
Text Solution
|
- AB(2) dissociates as AB(2(g)) Leftrightarrow AB((g))+B((g)) . Whwn the...
Text Solution
|
- For the reaction AB(2(g)) hArr AB((g)) + B((g)) if alpha is negligiabl...
Text Solution
|