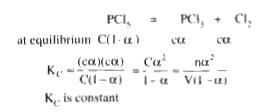A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- PCI5 dissociates to 25% in a three litre flask. To what volume the co...
Text Solution
|
- The degree of dissociation of PCl(5) at 1 atm pressure is 0.2. Calcula...
Text Solution
|
- In an evacuated vessel of capacity 110 litres, 4moles of Argon and 5 m...
Text Solution
|
- The dissociation of PCI5 decreases in presence of CI2 Why ?
Text Solution
|
- The dissociation of HI independent of pressure, while dissociation of ...
Text Solution
|
- PCI5(g) at an initial pressure of 1 atm is placed in a closed vessel c...
Text Solution
|
- Two moles of PCI5 were heated to 327^@C in a closed two litre vessel a...
Text Solution
|
- At temperature T k PCI5 is 50% dissociated at an equilibrium pressure ...
Text Solution
|
- 4.0 moles of PCI5 dissociated at 760 K in a 2 litre flask PCI5 (g) hAr...
Text Solution
|