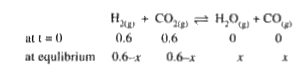A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- At a given temperature, Ke is 4 for the reaction H(2(g)) + CO(2(g)) hA...
Text Solution
|
- For the reaction CO(g)+H(2)O(g) hArr CO(2)(g)+H(2)(g) at a given tempe...
Text Solution
|
- For the reaction CO(g)+H(2)O(g) hArr CO(2)(g)+H(2)(g) at a given tempe...
Text Solution
|
- Delta(f)^(@) for CO(2(g)), CO((g)) and H(2)O((g)) are -393.5,-110.5 an...
Text Solution
|
- Which of the following graphs are correct for the given reaction? H(...
Text Solution
|
- For the reaction, CO((g))+H(2)O((g))hArrCO(2(g))+H(2(g)) at a given te...
Text Solution
|
- For the reaction CO(g) +H(2)O(g) hArr CO(2)(g)+H(2)(g) at a given ...
Text Solution
|
- Calculate DeltaHf^@ for the reaction CO2(g) +H2(g) to CO(g) + H2O(g) g...
Text Solution
|
- For the equilibrium H(2)(g)+CO(2)(g)hArr hArr H(2)O(g)+CO(g), K(c)=16 ...
Text Solution
|