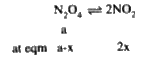A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- N(2)O(4) harr 2NO(2), N(2)O(4(g)) dissociates until the partial pressu...
Text Solution
|
- N(2)O(4) dissociates as N(2)O(4)(g)hArr2NO(2)(g) At 40^(@)C and on...
Text Solution
|
- When NO and NO(2) are mixed, the following equilibria are readily obta...
Text Solution
|
- The degree of dissociation of N(2)O(4)(1) obeying the equilibrium, N...
Text Solution
|
- In the reaction N(2)O(4) hArr 2NO(2) , the degree of dissociation of N...
Text Solution
|
- अभिक्रिया, N(2)O(4)(g) ltimplies 2NO(2)(g) के साम्य सिथरांक की गड़ना क...
Text Solution
|
- At 46° C Kp for the reaction N(2)O(4) (g)hArr 2NO(2)(g) is 0.667 atm. ...
Text Solution
|
- For the reaction N(2)O(4)hArr 2NO(2(g)), the degree of dissociation of...
Text Solution
|
- K(p) for the reaction N(2) O(4) (g) rarr 2NO(2)(g) is 0.66 at 46^(@)C....
Text Solution
|