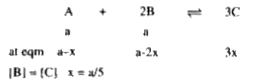A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The initial concentrations of A and B are same for A +2B harr 3C and w...
Text Solution
|
- In an equilibrium A+B hArr C+D, A and B are mixed in vessel at tempera...
Text Solution
|
- For the reaction A+B hArr C+D , the initial concentrations of A and B ...
Text Solution
|
- A(g) + 3B(g) ltimplies 4C(g), A का प्रारंभिक सांद्रण B के समतुल्य है A...
Text Solution
|
- In an equilibrium A+BhArrC+D,A and B are mixed in a vessel at temperat...
Text Solution
|
- For the reaction A + 2B hArr 2C + D, initial concentration of A is a a...
Text Solution
|
- In a system A(s) hArr 2B(g) +3C (g) if the conc. Of C at equilibrium...
Text Solution
|
- अभिक्रिया a + b hArr c+ d के लिए, a व b की प्रारम्भिक सांद्रताएँ ब...
Text Solution
|
- A(g) + 3(g) hArr 4C(g) Initial concentration of A is equal to that of ...
Text Solution
|