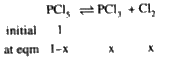A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- PCl(5) dissociates as follows in a closed reaction vessel PCI(5(g)) ha...
Text Solution
|
- In the dissociation of PCl(5) as PCl(5)(g) hArr PCl(3)(g)+Cl(2)(g) If ...
Text Solution
|
- 1 mol of Cl(2) and 3 mol of PCl(5) are placed in a 100 L vessel heated...
Text Solution
|
- PCl(5) dissociation a closed container as : PCl(5(g))hArrPCl(3(g))+Cl(...
Text Solution
|
- Phosphorous pentachloride dissociates as follows (in a closed reaction...
Text Solution
|
- PCl(5)(g) hArr PCl(3)(g)+Cl(2)(g) 'alpha' is the degree of dissociatio...
Text Solution
|
- Phosphorus pentachloride dissociates as follows, ina closed reaction v...
Text Solution
|
- a' moles of PCl(5) are heated in a closed container to equilibrate PCl...
Text Solution
|
- एक बंद पात्र में फॉस्फोरस पेटाक्लोराइड का वियोजन इस प्रकार होता हैं: ...
Text Solution
|