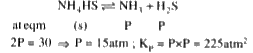A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the following reaction NH(4)HS((s)) harr NH(3(g))+H(2)S((g)) the t...
Text Solution
|
- For NH(4)HS(s) hArr NH(3)(g)+H(2)S(g) , the observed, pressure for rea...
Text Solution
|
- Some acid NH(4)HS is placed in flask containing 0.5 atm of NH(3). What...
Text Solution
|
- On decomposition of NH(4)HS , the following equilibrium is estabilishe...
Text Solution
|
- NH(4)HS(s)hArrNH(3(g))+H(2)S(g) The equilibrium pressure at 25(@) C is...
Text Solution
|
- Some acid NH(4)HS is placed in flask containing 0.5 atm of NH(3). What...
Text Solution
|
- Calculate K(p) for the equilibrium, NH(4)HS((s))hArrNH(3(g))+H(2)S((g)...
Text Solution
|
- For the following reaction : NH(2) COO NH(4) (s) hArr 2 NH(3) (g) + ...
Text Solution
|
- The equilibrium NH(4)HS(s)hArr NH(3)(g)+H(2)S(g), is followed to set -...
Text Solution
|