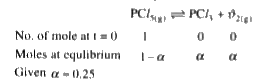A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- At a certain temperature, the degree of dissociation of PCI(5) was fou...
Text Solution
|
- K(p) for the reaction PCl(5)(g)ghArrPCl(3)(g)+Cl(2)(g) at 250^(@)C is ...
Text Solution
|
- At some temperature and under a pressure of 4 atm, PCl(5) is 10% disso...
Text Solution
|
- Calculate the volume percent of chlorine gas at equilibrium in the dis...
Text Solution
|
- The degree of dissociation of PCl(5) at 1 atm pressure is 0.2 . Calcul...
Text Solution
|
- the degree of dissociation of PCI(5) at a certain temperature and und...
Text Solution
|
- At some temperature and under a pressure of 4 atm, PCl(5) is 10% disso...
Text Solution
|
- The equilibrium constant K(p) for the thermal dissociation of PCl(5) a...
Text Solution
|
- K(p) for the reaction PCl(5)(g)ghArrPCl(3)(g)+Cl(2)(g) at 250^(@)C...
Text Solution
|