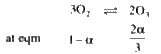A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 3O(2) harr 2O(3). Oxygen is ozonised until the partial pressures of bo...
Text Solution
|
- At 25^(@)C and 1 atm pressure, the partial pressure in equilibrium mix...
Text Solution
|
- Calculate the equilibrium constant K(p) and K(c ) for the reaction: CO...
Text Solution
|
- The equilibrium constant K(p(2)) and K(p(2)) for the reactions A hArr ...
Text Solution
|
- For the reaction A(g) + B(g) hArr C(g) at equilibrium the partial pres...
Text Solution
|
- Calculate the equilibrium constants K(p) and K(c) for the reaction , C...
Text Solution
|
- अभिक्रिया 2SO(2) + O(2) hArr 2SO(3) के लिए 727^(@)C ताप पर साम्य स्थिर...
Text Solution
|
- The reaction : 3O(2)hArr 2O(2), DeltaH=+69,000 calories is aided by :
Text Solution
|
- For the reaction C(s) +CO(2)(g) rarr 2CO(g), k(p)=63 atm at 100 K. If ...
Text Solution
|