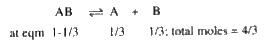Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the equilibrium AB((g)) harr A((g)) +B((g)), at a given temperatur...
Text Solution
|
- AB(2) dissociates as AB(2)(g) hArr AB(g)+B(g) . If the initial pressur...
Text Solution
|
- For the equilibrium AB(g) hArr A(g)+B(g) at a given temperature, the p...
Text Solution
|
- AB(3)(g) is dissociation as AB(2)(g) hArr AB(2)(g)+(1)/(2)B(2)(g), W...
Text Solution
|
- AB(2) " dissociates as " : AB(2) (g) hArr AB (g) + B (g). If the intia...
Text Solution
|
- The dissociation equilibrium of a gas AB2 can be represented as, 2AB2(...
Text Solution
|
- The dissociation of a gas AB(2) at equilibrium can be represented as :...
Text Solution
|
- The dissociation equilibrium of AB(2) gas is, 2AB(2)(g) hArr 2AB(g)+...
Text Solution
|
- The following reaction is at equilibrium at a particular temperature: ...
Text Solution
|