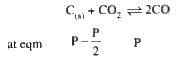Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the reaction, C((s)) + CO(2(g)) harr 2CO((g)) the equilibrium press...
Text Solution
|
- In the reaction C(s)+CO(2)(g) hArr 2CO(g) , the equilibrium pressure i...
Text Solution
|
- In the reaction C((s))+CO(2(g))hArr2CO((g)), the equilibrium pressure ...
Text Solution
|
- If 50% of CO(2) converts to CO at the following equilibrium : 1/2C(s) ...
Text Solution
|
- In the reaction C(s)+CO(2)(g) hArr 2CO(g), the equilibrium pressure is...
Text Solution
|
- अभिक्रिया CO(g)+(1)/(2)O2(g)hArr CO(2)(g) के लिए K(p)//K(c) का मान होत...
Text Solution
|
- For the reaction, 0.5 C(s)+0.5CO(2)(g) hArr CO(g) the equilibrium pres...
Text Solution
|
- NH(2)COONH(2)(s) harr 2NH(3)(g) + CO(2)(g) If equilibrium pressure is ...
Text Solution
|
- For the reaction C(s) + CO(2) (g) hArr 2CO(g). the partial pressures o...
Text Solution
|