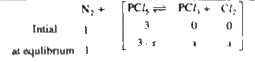Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- One mole of N(2) and 3 mole of PCI(5) are placed in a 100 litre vessel...
Text Solution
|
- 1 mol of Cl(2) and 3 mol of PCl(5) are placed in a 100 L vessel heated...
Text Solution
|
- In the reaction, PCl(5)hArrPCl(3)+Cl(2), the amounts of PCl(5), PCl(3)...
Text Solution
|
- n mole of PCl(3) and n mole of Cl(2) are allowed to react at constant ...
Text Solution
|
- 1 mole of PCl(5) taken at 5 atm, dissociates into PCl(3) and Cl(2) to...
Text Solution
|
- One moel of N(2) and 3.0 moles of PCl(5) were placed in a 100-lotre ve...
Text Solution
|
- One mole of N(2) and 3.0 moles of PCl(5) were placedin a 100-liter ves...
Text Solution
|
- 30 moles of PCl(5) are placed in a 2 L reaction vessel and heated. K(c...
Text Solution
|
- A quantity of PCl(5) ws heated in a 10 litre vessel at 250^(@)C: PCl(5...
Text Solution
|