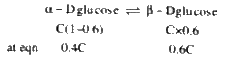A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- alpha - D glucose undergoes mütarotation to beta-D glucose in an aqueo...
Text Solution
|
- alpha-D (+)- glucose and beta-D(+)- glucose are:
Text Solution
|
- alpha -D(+)-glucose and beta -D(+) glucose are
Text Solution
|
- alpha - D -(+)- glucose and beta-D-(+)- glucose are
Text Solution
|
- alpha-(D)" glucose "hArr beta-(D) glucose, equilibrium constant for th...
Text Solution
|
- alpha D(+) Glucose and beta D (+) glucose are
Text Solution
|
- alpha -D-Glucose and beta -D-Glucose are:
Text Solution
|
- alpha - D (+) Glucose and beta - D (+) glucose are ……… .
Text Solution
|
- alpha -D(+) glucose and beta-D(+) glucose are :
Text Solution
|