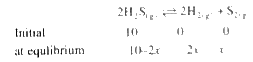A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- H(2)S((g)) intially at a pressure of 10 atm anda temperature of 800K,...
Text Solution
|
- Calculate K(c) for the reaction: 2H(2)(g)+S(2)(g) hArr 2H(2)S(g) if 1....
Text Solution
|
- Equilibrium constant K(p) for H(2)S(g) hArr 2H(2)(g)+S(2)(g) is 0.0118...
Text Solution
|
- Equilibrium constant (K(p)) for 2H(2)S((g))hArr2H(2(g))+S(2(g)) is 0.0...
Text Solution
|
- An equilibrium mixture for the reaction 2H(2)S(g) hArr 2H(2)(g) + S(...
Text Solution
|
- When S in the form of S(g) is heated at 1000K, the initital pressure o...
Text Solution
|
- The equilibrium pressure for the reaction MSO(4).2H(2)O (s) hArr MSO(4...
Text Solution
|
- The equilibrium constant for, 2H(2)S (g)hArr 2H(2)(g) + S(2)(g) is 0.0...
Text Solution
|
- The K(p) of the reaction is NH(4)HS((s)) Leftrightarrow NH(3(g))+H(2)S...
Text Solution
|