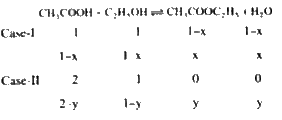A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- On taking 60 g CH(3)COOH and 46g CH(3)CH(2)OH in a 5 lit. lask in tle ...
Text Solution
|
- Arrange the following compounds in the increasing order of their boili...
Text Solution
|
- 60g CH(3)COOH and 46g C(2)H(5)OH react in 5L flask to form 44g CH(3)CO...
Text Solution
|
- Amongest the following maximum number of compounds which give iodofor...
Text Solution
|
- निम्नलिहित यौगिकों को कवथनांकों के बढ़ते कर्म में व्यवस्थित कीजिए - C...
Text Solution
|
- CH(3)-COOH+CH(3)-OH to CH(3)-COO-CH(3)+H(2)O
Text Solution
|
- Reaction CH(3)COOH+C(2)H(5)OH to CH(3)COOC(2)H+H(2)O is an illustratio...
Text Solution
|
- Arrange the following compounds in the increasing order of their boili...
Text Solution
|
- The number of alcohols giving iodoform test among the following is: ...
Text Solution
|