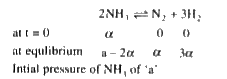A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Ammonia under a pressure of 1.5 atm at 27^(@)C is heated to 374^(@)C i...
Text Solution
|
- Ammonia under a pressure of 15 atm , at 27^(@)C is heated to 327^(@)C ...
Text Solution
|
- NH(3) is heated at 15 at, from 25^(@)C to 347^(@)C assuming volume con...
Text Solution
|
- For the equilibrium N(2)+3H(2) hArr 2NH(3) at 298K and 5 atm. Pressure...
Text Solution
|
- 2NH(3)hArrN(2)+3H(2). the vessel is such that the volume remains effec...
Text Solution
|
- NH(3) is heated at 15 at, from 25^(@)C to 347^(@)C assuming volume con...
Text Solution
|
- In the following reaction we start with 2 mol of N(2) and 5 of H(2) e...
Text Solution
|
- In the folowing reaction, we start with 2 mol of N(2) and 5 mol of H(2...
Text Solution
|
- Ammonia under a pressure of 1.5 atm at 27^(@)C is heated to 374^(@)C i...
Text Solution
|