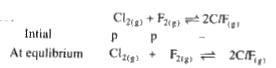A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Equal moles of F(2(g)) and Cl(2(g)) are introduced into a sealed cont...
Text Solution
|
- For the reaction 2CI F(3)(g) harr CI(2)(g) +3F(2)(g) log K(eq) vs (1...
Text Solution
|
- The exothermic formation of CiF(3) is represented by the equation Cl(2...
Text Solution
|
- The following two equilibria exist simultaneously in a closed vessel :...
Text Solution
|
- CIF(3) का ऊष्माक्षेपी निर्माण निम्न अभिक्रिया द्वारा प्रदर्शित किया जा...
Text Solution
|
- What is the order for the following reactions ? (a) 2NO(2(g)) ...
Text Solution
|
- In the reaction, 2" NO"(2)(g)+F(2)(g)to2" NO"(2)F(g), order with respe...
Text Solution
|
- For the reaction Cl(2)(g)+F(2)(g)hArrClF(g),K(c)=19.9 What will happen...
Text Solution
|
- At 27^(@)C NO and Cl(2) gases are introduced in a 10 litre flask such ...
Text Solution
|