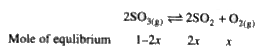Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- One lit of SO(3) was placed in a two litre vessels of a certain tempe...
Text Solution
|
- One mole of SO(3) was placed in a litre reaction flask at a given temp...
Text Solution
|
- One mole of SO(3) was placed in a two litre vessel at a certain temper...
Text Solution
|
- A vessel of one litre capacity containing 1 mole of SO(3) is heated ti...
Text Solution
|
- SO(2)निहित दो गैसीय साम्य तथा 298K पर उनके सांगत साम्यवास्था स्थिरांक ...
Text Solution
|
- निम्न साम्य के लिए Kc क्या होगा यदि साम्य पर प्रत्येक पदार्थ की सांद्र...
Text Solution
|
- At equilibrium degree of dissociation of SO(3) is 50% for the reaction...
Text Solution
|
- One mole of SO(3) was placed in a litre reaction vessel at a certain t...
Text Solution
|
- For the reaction : 2 NH(3)(g) rarr N(2)(g) + 3 H(2)(g)
Text Solution
|