A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMS
AAKASH SERIES|Exercise EXERCISE -IB|44 VideosATOMS
AAKASH SERIES|Exercise EXERCISE -II|35 VideosATOMS
AAKASH SERIES|Exercise PRACTICE EXERCISE|21 VideosAPPENDICES (REVISION EXERCISE)
AAKASH SERIES|Exercise LAW OF MOTION|91 VideosCAPACITORS
AAKASH SERIES|Exercise PRACTICE SHEET (ADVANCED) (Integer Type Questions)|2 Videos
AAKASH SERIES-ATOMS-EXERCISE -IA
- When electrons jump from higher energy orbits to third energy orbit sp...
Text Solution
|
- The energy of electron in the ground state of Hydrogen atom is -13. 6e...
Text Solution
|
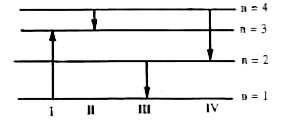
- The diagram shows the energy levels for an electron iin a certain atom...
Text Solution
|
- The wavelengths involved in the specturm of deuterium (""(1)D^(2)) are...
Text Solution
|
- Atomic hydrogen is excited from the grounds state to the n^(th) state....
Text Solution
|
- Which of the following is not correct in Bohr model of hydrogen atom ?
Text Solution
|
- In the Bohr model of a hydrogen atom, the centripetal force is furnish...
Text Solution
|
- The Bohr's model of atoms:
Text Solution
|
- Bohr's model for the hydrogen atom predicts that the absorption spectr...
Text Solution
|
- Three photons coming from excited atomic hydrogen sample are picked up...
Text Solution
|
- Whenever a hydrogen atom emits a photon in the Balmer series
Text Solution
|
- If n gt gt 1, then the dependence of frequency of photon emitted as a ...
Text Solution
|
- When a hydrogen atom is raised from the ground state to fifth stale(ex...
Text Solution
|
- Consider a spectral line resulting from the transition n = 5 to n = 1,...
Text Solution
|
- In which of the following systems will the radius of the first orbit (...
Text Solution
|
- If the radius of an orbit is r and the velocity of electron in it is v...
Text Solution
|
- As the quantum number increases, the difference of energy between cons...
Text Solution
|
- In which of the following transitions will the wavelength be minimum ?
Text Solution
|
- Which of the following parameters are the same for all hydrogen-like a...
Text Solution
|
- The angular momentum of an electron in a hydrogen atom is proportion t...
Text Solution
|