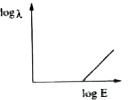
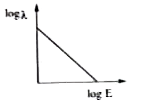
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The graph between momentum p and de-Broglie wavelength lambda of photo...
Text Solution
|
- The de Broglie wavelength of an electron and the wavelength of a photo...
Text Solution
|
- Momentum of a photon of wavelength lambda is :
Text Solution
|
- de-Broglie wavelength lambda is
Text Solution
|
- सिद्ध कीजिए कि lambda तरंगदैर्घ्य के फोटॉन का संवेग p = h//lambda हो...
Text Solution
|
- The momentum of a photon of wavelength lambda is
Text Solution
|
- Draw the curve showing the variation of de Broglie wavelength of a par...
Text Solution
|
- Plot a graph of the de-Broglie wavelength associated with a photon ver...
Text Solution
|
- Plot a graph of the de - Broglie wavelength associated with a proton v...
Text Solution
|