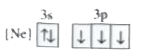
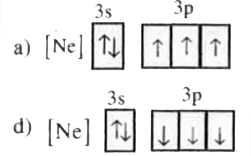
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Ground state electronic configuration of p atom can be represented as
Text Solution
|
- Which of the following can be acceptable electronic configuration of c...
Text Solution
|
- Which of the following options does not represent ground state electr...
Text Solution
|
- Ground state electronic configuration of nitrogen atom can be represen...
Text Solution
|
- The ground state electronic configuration of nitrogen atom can be repr...
Text Solution
|
- Ground state electronic configuration of nitrogen atom can be represen...
Text Solution
|
- Ground state electronic configuration of nitrogen atom can be represen...
Text Solution
|
- Which of the following options does not represent ground state electro...
Text Solution
|
- Which of the following do not represent ground state electronic config...
Text Solution
|