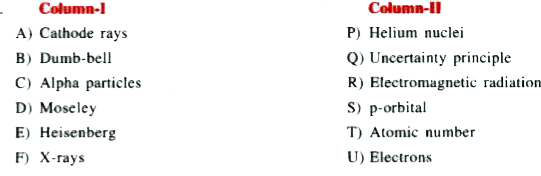Text Solution
Verified by Experts
The correct Answer is:
Recommended Questions
- Match the following
Text Solution
|
- Match the following The correct match is
Text Solution
|
- Match the following Match correc match is
Text Solution
|
- Match the following : The correct match is
Text Solution
|
- Match the following : The correct match is
Text Solution
|
- Match the following . The correct match is .
Text Solution
|
- निम्न को सुमेलित करिए सही सुमेल है
Text Solution
|
- Match the following the correct match is
Text Solution
|
- Match the following The correct match is
Text Solution
|