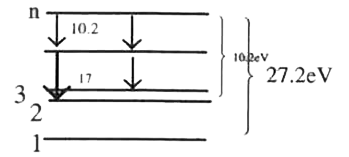
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A hydrogen-like atom (atomic number Z) is in a higher excited state of...
Text Solution
|
- A hydrogen -like atom (atomic number Z) is in a higher excited state c...
Text Solution
|
- A hydrogen like atom (atomic number Z) is in a higher excited satte ...
Text Solution
|
- A hydrogen like atom (atomic number Z) is in a higher excited satte ...
Text Solution
|
- A hydrogen like atom (atomic number Z) is in a higher excited satte of...
Text Solution
|
- A hydrogen like atom (atomic number Z) is in a higher excited satte of...
Text Solution
|
- A hydrogen like atom (atomic number = Z) is in higher excited state of...
Text Solution
|
- A hydrogen like species with atomic number 'Z' is in higher excited st...
Text Solution
|
- A hydrogen like species (atomic number Z) is present in a higher excit...
Text Solution
|