A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE - II) (LEVEL - II (ADVANCED)) (Linked Comprehension Type Questions)|9 VideosREDOX REACTIONS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE - II) (LEVEL - II (ADVANCED)) (Matrix Matching Type Questions)|2 VideosREDOX REACTIONS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE - II) (LEVEL - II (ADVANCED)) (Straight Objective Type Questions)|15 VideosPURIFICATION OF ORGANIC COMPOUNDS AND IUPAC NOMENCLATURE
AAKASH SERIES|Exercise ADDITIONAL PRCATICE EXERCISE (LEVEL - II (LECTURE SHEET (ADVANCED) INTEGER TYPE QUESTIONS)|2 VideosREVISION EXERCISE
AAKASH SERIES|Exercise CHEMICAL EQUILIBRIUM|107 Videos
AAKASH SERIES-REDOX REACTIONS-LECTURE SHEET (EXERCISE - II) (LEVEL - II (ADVANCED)) (More than One correct answer Type Questions)
- The equation for a reaction is shown below: 2MnO(4)^(-) + 5H(2)O2+ 6H^...
Text Solution
|
- 5Cu + 2HNO(3)rarr 5CuO + H(2)O + N(2), mark out the correct statement(...
Text Solution
|
- On being heated in oxygen, 3.120 g of a metal M converts to 4.560 g of...
Text Solution
|
- Which of the following are correct about the reaction, FeS(2) + O(2)ra...
Text Solution
|
- Preparation of Cl2 from HCl and MnO2 involves the process of:
Text Solution
|
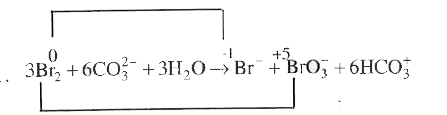
- In the reaction 3Br(2) + 6CO(3)^(2-) + 3H(2)OrarrBr^(-) + BrO3 + 6HCO...
Text Solution
|
- The salt KHC(2)O(4)H(2)C(2)O(4).4H(2)O may be used as reducing agent ...
Text Solution
|