A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - I) ( LEVEL-II (ADVANCED))(Linked Comprehension Type Questions)|6 VideosREDOX REACTIONS
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - I) ( LEVEL-II (ADVANCED))(Matrix Matching Type Questions)|1 VideosREDOX REACTIONS
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - I) ( LEVEL-II (ADVANCED))(Straight Objective Type Questions )|33 VideosPURIFICATION OF ORGANIC COMPOUNDS AND IUPAC NOMENCLATURE
AAKASH SERIES|Exercise ADDITIONAL PRCATICE EXERCISE (LEVEL - II (LECTURE SHEET (ADVANCED) INTEGER TYPE QUESTIONS)|2 VideosREVISION EXERCISE
AAKASH SERIES|Exercise CHEMICAL EQUILIBRIUM|107 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-REDOX REACTIONS-PRACTICE SHEET (EXERCISE - I) ( LEVEL-II (ADVANCED))(More than One correct answer Type Questions)
- Which statement(s) about oxidation number is(are) correct?
Text Solution
|
- Among the following statements the correct are :
Text Solution
|
- The process reduction. involves
Text Solution
|
- Indicate in which of the following processes nitrogen is recuced ?
Text Solution
|
- Which of the following statements (s) is (are) correct ?
Text Solution
|
- Which of the following reaction() is (are) not oxidation, reduction ?
Text Solution
|
- Which of the following represents redox reaction ?
Text Solution
|
- Which is (are) disproportionation reaction(s)?
Text Solution
|
- Which among the following are auto - redox reactions ?
Text Solution
|
- Thermal decomposition of (NH(4))(2)Cr(2)O(7) involves.
Text Solution
|
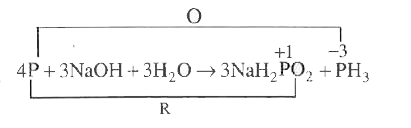
- When P reacts with caustic soda. The products are PH(3) and NaH(2)PO(2...
Text Solution
|