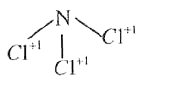A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II (LECTURE SHEET (ADVANCED) (More than One correct answer Type Questions)|11 VideosREDOX REACTIONS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II (LECTURE SHEET (ADVANCED) (Linked Comprehension Type Questions)|6 VideosREDOX REACTIONS
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - II) ( LEVEL-II) (ADVANCED))(Matrix Matching Type Questions)|7 VideosPURIFICATION OF ORGANIC COMPOUNDS AND IUPAC NOMENCLATURE
AAKASH SERIES|Exercise ADDITIONAL PRCATICE EXERCISE (LEVEL - II (LECTURE SHEET (ADVANCED) INTEGER TYPE QUESTIONS)|2 VideosREVISION EXERCISE
AAKASH SERIES|Exercise CHEMICAL EQUILIBRIUM|107 Videos
AAKASH SERIES-REDOX REACTIONS-ADDITIONAL PRACTICE EXERCISE (LEVEL - I (MAIN)) (Straight Objective Type Questions)
- Chlorine is passed into dilute cold KOH solution. What are the oxidati...
Text Solution
|
- What is the oxidation number of sulphur in Na(2)S(4)O(6)?
Text Solution
|
- The oxidation number of nitrogen in NCI(3) is
Text Solution
|
- What are the oxidation numbers of 'N' in NH(4)NO(3)?
Text Solution
|
- Iron has the lowest oxidation state in
Text Solution
|
- The oxidation number of phosphorus in sodium hypophosphite is
Text Solution
|
- PbS+4H(2)O(2)rarrPbSO(4)+4H(2)O. In this reaction PbS undergoes
Text Solution
|
- MnO(2)+4HClrarrMnCl(2)+Cl(2)+2H(2)O, In the reaction MnO(2) acts as
Text Solution
|
- In the reaction P(4)+3OH^(-)+3H(2)Orarr 3H(2)PO(2)^(-)+PH(3) phosp...
Text Solution
|
- Among the following ion the one that cannot undergo disproportionation
Text Solution
|
- The reaction is Decomposition but it's not redox reaction
Text Solution
|
- Which of the following is redox reaction
Text Solution
|
- Which of the following is metal displacement reaction
Text Solution
|
- Which of the following is a redox reaction?
Text Solution
|
- Which of the following is not an example of disproportionation reactio...
Text Solution
|
- In the reaction 3Mg+N(2)rarrMg(3)N(2)
Text Solution
|
- Which one of the halogn is prepared by only electrolysis method
Text Solution
|
- Which of the following statement is correct for a galvanic cell?
Text Solution
|
- In Cu - Zn cell
Text Solution
|
- In a electrochemical cell
Text Solution
|