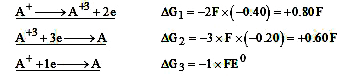A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Given that the standard oxidation potentials (E^0) of A|A^(+3) and A^(...
Text Solution
|
- Given standard electrode potentials: Fe^(3+)+3e^(-)toFe,E^(@)=-0.036...
Text Solution
|
- Given that the standard reduction potentials E ^(0) of Fe^(+2)|Feis 0....
Text Solution
|
- Given that the standard reduction potential (E^(@)) of Cr^(3+) | Cr an...
Text Solution
|
- Given that the standard oxidation potentials (E^0) of A|A^(+3) and A^(...
Text Solution
|
- Given : Co^(3+) + e^(-) to Co^(2+) , E^(@) = +1.81V pb^(4+) + 2e^(-) r...
Text Solution
|
- Predict whether Tit will disproportionate in aqueous solution, given t...
Text Solution
|
- বিজারণ বিভবের ভিত্তিতে ক্রমহ্রাসমান জারণ ক্ষমতা অনুসারে সাজাও- আয়ন (C...
Text Solution
|
- Standard electrode potentials of Fe^(2+) + 2e to Fe and Fe^(3+) + 3e t...
Text Solution
|