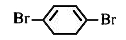
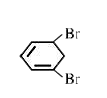
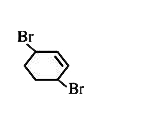
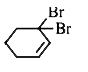A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- What is the final product of the following reaction sequence? Cycloh...
Text Solution
|
- The final product of the following reaction is COCH3overset(Delta)toov...
Text Solution
|
- The major product of the following reaction is : overset((i)NaNO2//H^+...
Text Solution
|
- What is the final product of the following reaction sequence? cyclo...
Text Solution
|
- In the following sequence of reactions, identify the final product. Cy...
Text Solution
|
- ইথানলoverset(PBr3)rarrXoverset(Alc. KOH)rarrYoverset((i) H2SO4 Room Te...
Text Solution
|
- Consider the following sequence of reaction overset(1.Br(2)+KOH)u...
Text Solution
|
- What is the final product of the following reaction sequence? Cyclohex...
Text Solution
|
- In the following sequence of reaction , CH3CH2Br overset(KOH(alc))to X...
Text Solution
|