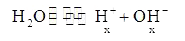A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Statement-1 : pH of 10^(-7) M HCl is less than 7 at 25^@C. Statemen...
Text Solution
|
- Assertion : pH of 10^(-7) M HCl is less than 7 at 25^(@)C. Reason : ...
Text Solution
|
- STATEMENT-1 : pH of water at 25^(@)C is less than the pH at 4^(@)C. ...
Text Solution
|
- Assertion (A): pH of HCI solution is less than that of acetic acid of ...
Text Solution
|
- कथन-1 -25^(@)C पर जल के आयनन की मात्रा बहुत-ही कम होती है। कथन-2 25^...
Text Solution
|
- Statement-1 : pH of 10^(-7) M HCl is less than 7 at 25^@C . Statement-...
Text Solution
|
- Determine the pH of the following solutions at 25^(@)C, (1) 10^(-7)(M)...
Text Solution
|
- What is the pH of 10^(-7)(M) HCl solution-
Text Solution
|
- What is the value pH pf 10^(-7) mol "litre"^(-1) HCl at 25^(@)C ?
Text Solution
|